
Metals are everywhere—in the wires in your walls, the pans in your kitchen, and even in your blood. These types of metal are defined by their atomic structure, properties, and how they interact with other elements.
Knowing the differences helps explain everything from rust resistance to why gold doesn’t tarnish.
All metals share certain traits: They’re electrically conductive, have a metallic luster, and a crystalline structure. But their chemical makeup and uses vary widely. Here’s a breakdown of the major categories.
1. Ferrous Metals

These contain iron and are known for their strength and magnetic properties. That includes carbon steel, wrought iron, and cast iron. They’re used in construction, tools, and automotive parts. But they’re more prone to rust unless alloyed for corrosion resistance.
2. Non-ferrous Metals
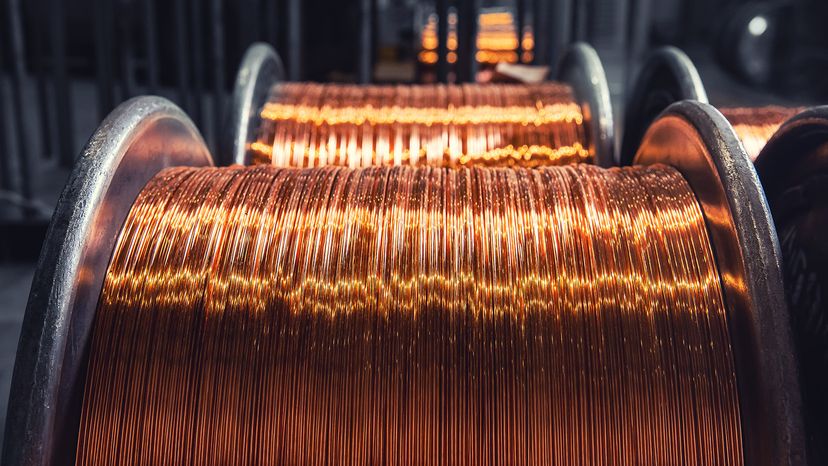
These don’t contain iron, so they’re naturally corrosion resistant and often lighter. Think: copper and aluminum. Their high electrical conductivity makes them essential in wiring and electronics. They’re also easier to shape and less brittle than many ferrous metals.
3. Stainless Steel

An iron carbon alloy with chromium added, stainless steel resists rust like a champ. Its corrosion-resistant properties make it ideal for marine hardware, medical tools, and kitchen equipment. It’s strong, shiny, and versatile.
4. Pure Metals

These are single-element materials like pure iron, copper, or aluminum. Found on the periodic table, they have similar chemical properties within groups.
They’re rarely used in their pure form for construction because they’re too soft, but they set the standard for thermal conductivity and electrical properties.
5. Precious Metals

Gold, silver, and the platinum group metals are both beautiful and functional. As noble metals, they don’t easily react with other elements.
Their high melting points, chemical stability, and resistance to corrosion make them valuable in electronics, jewelry, and the chemical industry.
6. Base Metals

Common and inexpensive, base metals like copper, nickel, and zinc are workhorses. They’re often combined to make metallic alloys such as copper alloys used in electrical wiring and heat exchangers. Despite being less glamorous, they’re essential to manufacturing.
7. Alkaline Earth Metals

Found in Group 2 of the periodic table, these include magnesium and calcium. They’re known for chemical reactivity and are crucial in everything from the aerospace industry to fireworks. Magnesium, a lightweight metal, is used in structural applications in the aerospace industry.
8. Alloyed Metals

When you create alloys, you’re blending metals for new unique properties. Adding alloying elements like chromium or vanadium changes tensile strength, heat resistance, and magnetic field interaction. Examples include high carbon steel, mild steel, and other specialized steel alloys.
9. Wrought Iron

Made by removing impurities from pig iron, wrought iron is tough yet malleable. Its low carbon content makes it resistant to fatigue, perfect for fences, railings, and decorative work. It’s not widely used today but still known for its workability.
10. Cast Iron

Unlike wrought iron, cast iron is hard and brittle. It contains more carbon and forms by pouring molten iron into molds. You’ll see it in cookware, pipes, and engine blocks. Its high thermal stability makes it ideal for heating applications.
We created this article in conjunction with AI technology, then made sure it was fact-checked and edited by a HowStuffWorks editor.




